1 Methodologies to Simulate Microgravity for Microbial Experiments
Hugo Castillo
Learning Objectives
-
Explain and compare microgravity simulation methods: Describe the physical principles, use-cases, and artifacts of low-shear modeled microgravity (HARV/RWV), clinostats, random positioning machines, and magnetic levitation.
-
Design and execute an experiment: Formulate a testable hypothesis (e.g., effects on growth or biofilm), select endpoints (OD600 growth curves, CFU counts, crystal-violet biofilm OD), prepare controls, and collect time-series samples with proper replication.
-
Analyze and interpret results: Estimate changes in growth rate and dynamics, on biofilms growth and development and on viability.
Introduction
Microgravity is a condition in which cells (and everything with mass) experience gravitational forces lower than those on Earth, making them appear weightless or floating. This occurs when cells are LEO, aboard the International Space Station (ISS) or during parabolic flights. Though gravity is still present, it is significantly reduced. For example, the gravitational force on Earth, the Moon and Mars is 9.81, 1.62 and 3.72 m/s2, respectively. In other words, if a cell on Earth weighs 100 ng, it would weigh 16.5 and 38 ng on the Moon and Mars.
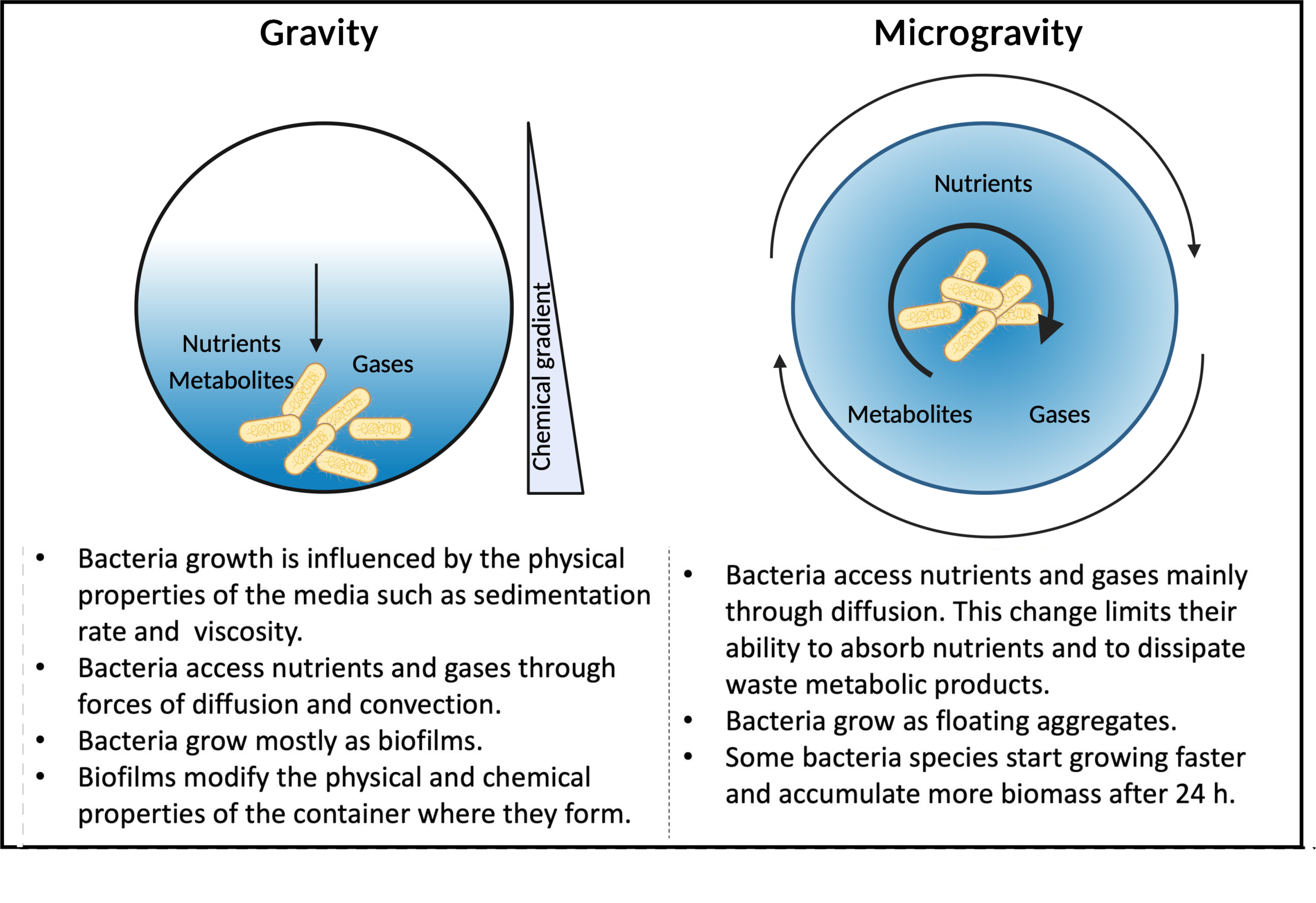
Studying the effects of microgravity on cells allows biologists to study how reduced gravitational forces change organisms in specific areas such as:
- Alteration of gene expression, protein production, and cell signaling pathways.
- Muscle atrophy, bone density loss, and cardiovascular adaptations on humans, which inform countermeasures for long-duration space missions and health applications on Earth.
- Changes in growth rates, biofilm formation, and antibiotic resistance in bacteria and fungi, impacting spaceflight safety and biotechnology.
- Plant growth adaptation in sustainable food production systems for deep-space exploration.
Microgravity analogs
Since conducting experiments in space is costly and logistically challenging, we use microgravity analogs on Earth to simulate weightless conditions and study bacterial responses. In other words, we simulate the effects of microgravity on cells, not microgravity itself. These models help investigate cell growth, gene expression, biofilm formation, and antibiotic resistance in bacteria under low-shear or altered gravity conditions.
- Rotating Wall Vessel (RWV) Bioreactors with Rotating Cell Culture Systems (RCCS)
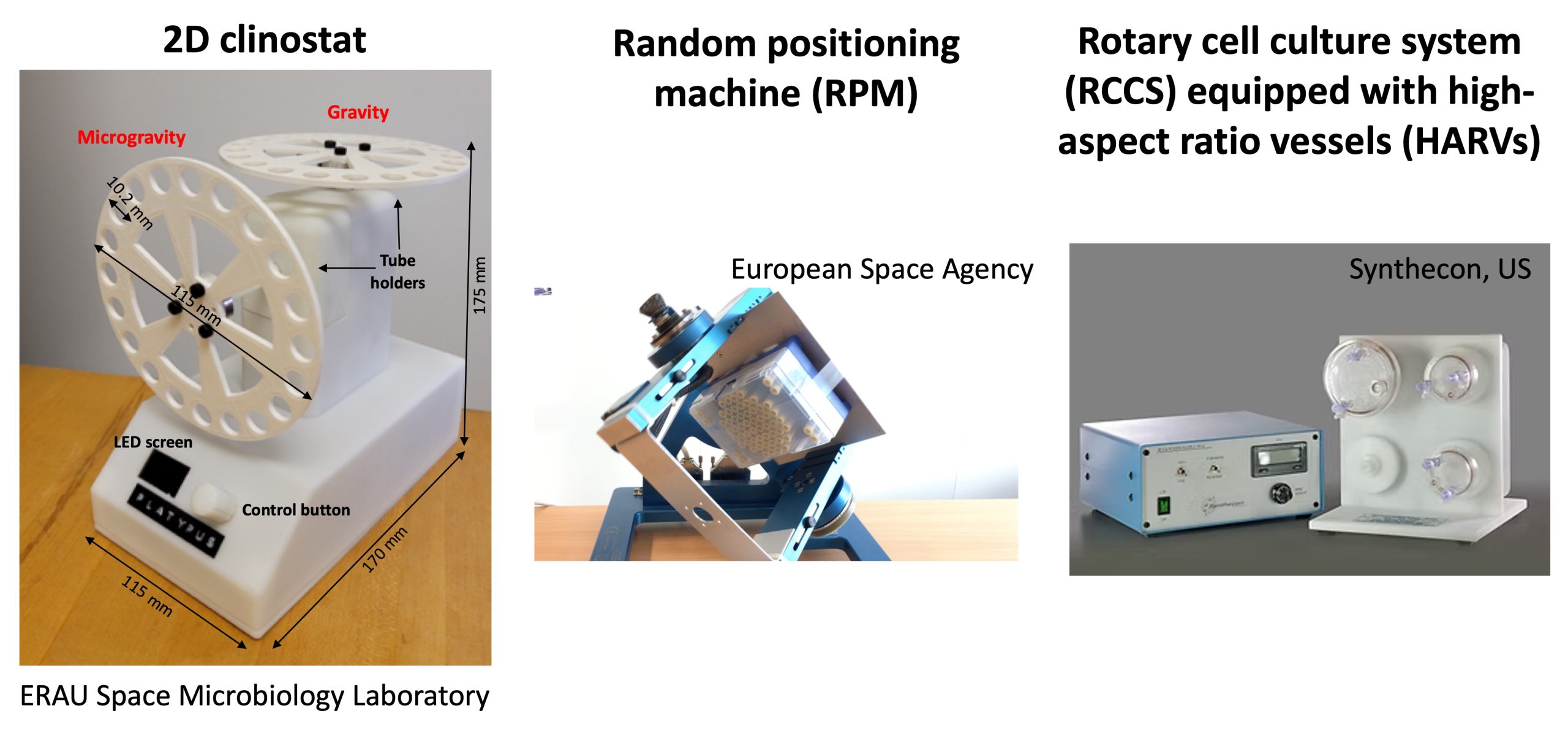
Developed by NASA, Rotating Wall Vessels (RWVs), also called High Aspect Ratio Vessels (HARVs) or Slow Rotating Lateral Vessels (SRLVs), simulate microgravity by continuously rotating a culture around a horizontal axis, keeping bacteria in suspension, reducing sedimentation and shear stress, mimicking space-like conditions. They are widely used because the low-shear fluid conditions used resemble the spaceflight environment. Multiple different studies have shown that cells grown with this system exhibit enhanced biofilm formation, cell aggregation, and changes in antibiotic resistance and pathogenicity.
- Clinostats
A clinostat rotates a sample along one or two axes to continuously change the direction of gravity’s pull, preventing the bacteria from sensing a fixed gravitational field. These devices are more affordable and simpler than RWV bioreactors, can be used for long-term experiments and in some cases allow researchers to run treatment and control conditions in parallel. They’re main application is to assess bacterial growth, motility, and gene expression.
- Random Positioning Machine (RPM)
The Random Positioning Machine (RPM) rotates a sample randomly along multiple axes, ensuring that the bacteria experience continuous changes in gravity vector direction. This instrument effectively reduces gravity to near 10⁻³ g, simulating long-term microgravity exposure. Compared to other analogs, it creates a more accurate simulation of microgravity conditions.
- Parabolic Flights
Aircraft (such as NASA’s “Vomit Comet” or ESA’s Zero-G Airbus) fly in parabolic arcs, creating brief periods (15-25 seconds) of microgravity during free fall. This is the closest Earth-based method to true microgravity in space and it allows direct comparison between normal gravity, hypergravity, and microgravity conditions.
- Magnetic Levitation
These analogs use superconducting magnets to create a force that counteracts Earth’s gravity, suspending bacterial cultures in a state that mimics weightlessness. One advantage over other analogs is that it provides weightlessness without rotation-induced shear stress. Although it is less commonly used for bacteria, it is being explored for cell biology studies.
Experimental Design
Define the research question or hypothesis.
Start with questions such as: How does microgravity affect bacterial growth rate and cell size? Do pathogenic bacteria become more virulent in microgravity? Does microgravity enhance bacterial biofilm formation? Are bacteria more resistant to antibiotics in space? Once you select your question, formulate a hypothesis such as: “When grown under simulated microgravity, Pseudomonas aeruginosa will form denser biofilms compared to normal gravity conditions”, “Escherichia coli becomes increases it resistance to antibiotics under simulated microgravity conditions” or “Clinorotation favors the growth of microaerophillic bacteria in saturated soils”.
Select the biological model and culture media
Once you have a hypothesis, identify which specie is more appropriate to test it. Start with those species with “space legacy”, this is, that have been previously used in real or simulated microgravity experiments. Consider other factors such as: Pathogenic bacteria (e.g. Salmonella typhimurium, Escherichia coli, Staphylococcus aureus), biofilm-forming bacteria (Pseudomonas aeruginosa, Klebsiella pneumoniae), spore-forming species (Bacillus subtilis) or extremophiles (Deinococcus radiodurans). Next identify the optimal growth conditions, starting with the growth media: Nutrient Broth/Agar for general-purpose bacterial growth, Luria-Bertani (LB) for enteric bacteria such as E. coli, urea-Based medium for Sporosarcina pasteurii, tryptic Soy Broth (TSB) + Glucose for biofilm studies, among others.
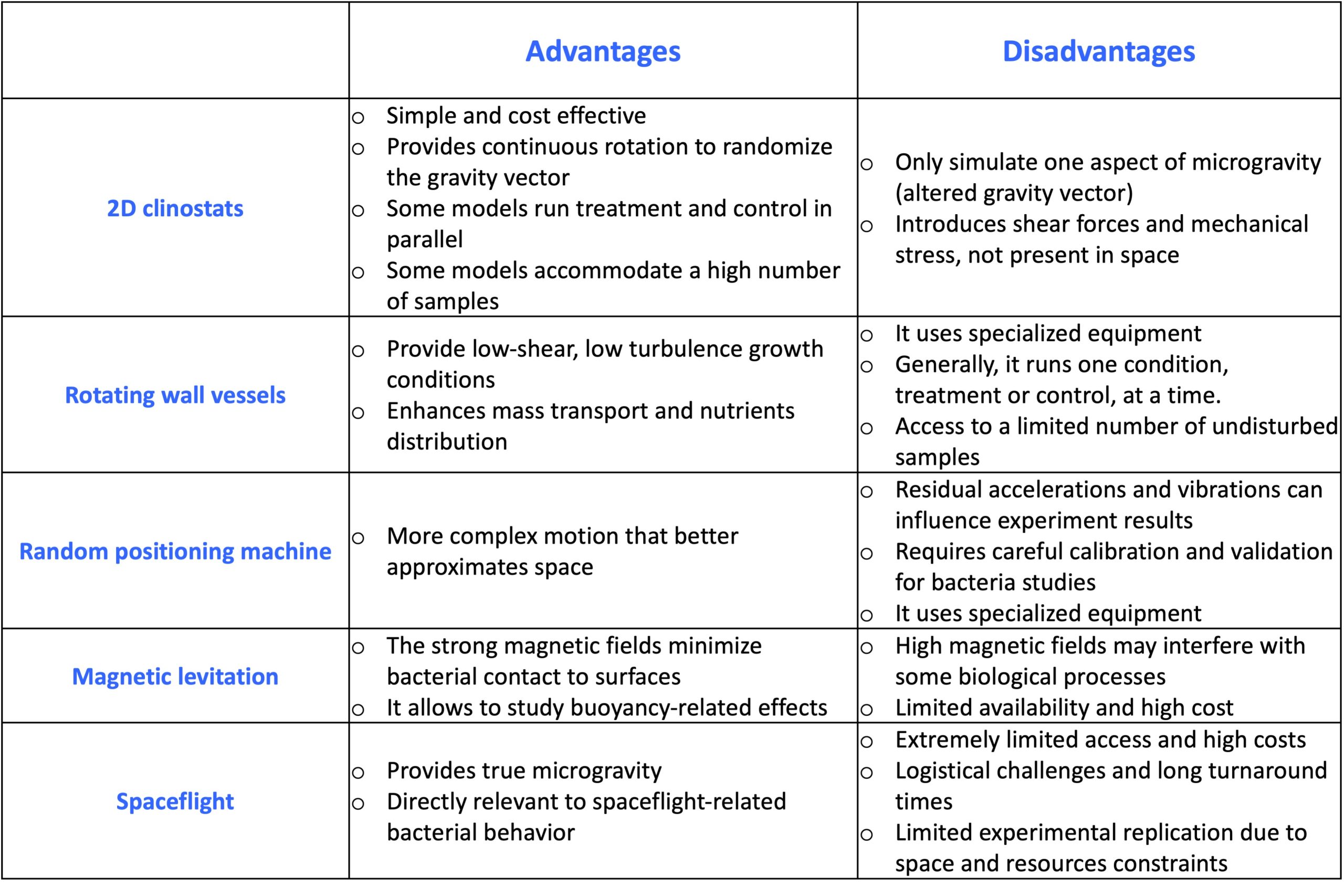
Selection of the microgravity analog
When choosing the analog for your experiments, consider the following advantages and disadvantages:
Experiment setup and sample preparation
Step 1: Culture Preparation
Inoculate bacterial cultures into sterile growth media and grow for ~ 15 h at its optimal temperature (30-37° C) under constant agitation. The day of the experiment, adjust the initial OD₆₀₀ (optical density at 600 nm) to ensure similar starting cell densities (~0.1–0.2 OD₆₀₀). Prepare the staring cell dilution (commonly 1:100) using the selected growth media, in triplicates, for each condition.
Step 2: Treatment (microgravity) and control (gravity) conditions
Transfer the bacteria suspensions to the appropriate growth vessel, depending on the analog used (HARVS for RWV or 2 mL screw-cap tubes for clinostats and RPM). Load the microgravity samples onto the device. For the control group, incubate the samples either under static conditions or onto the clinostat gravity position, if available.
Step 3: Incubation
Incubate both treatment and control under identical temperature, oxygen, and media conditions. Monitor growth for 24–48 hours or longer, depending on bacterial doubling time and the type of information you need to test your hypotheses.
Biological endpoints
The potential effects of simulated microgravity on bacteria cultures could be measured using different biological endpoints, based on the hypotheses defined:
- Growth Kinetics and viability
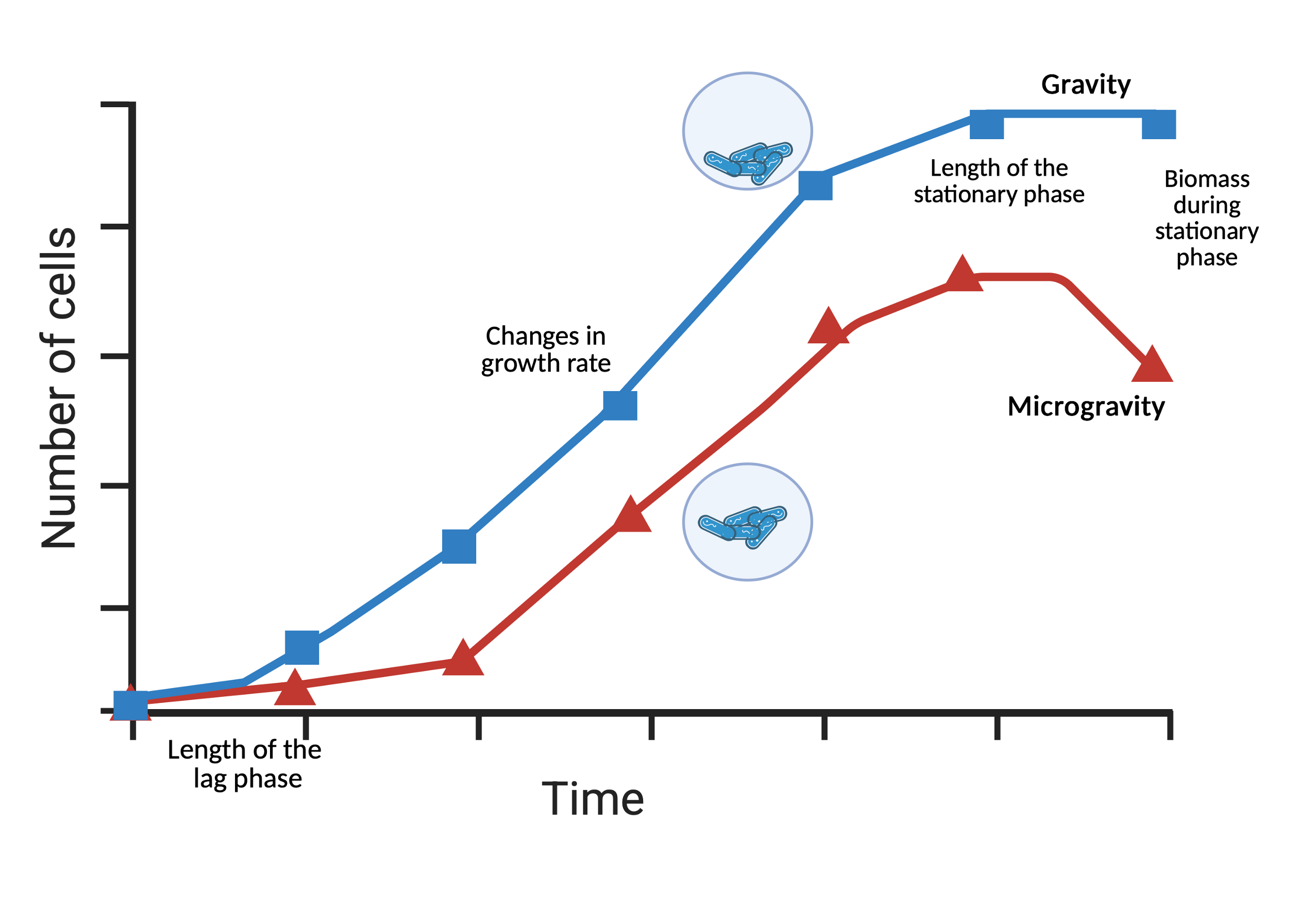
The most straightforward parameter for estimating bacterial growth is optical density at 600 nm (OD₆₀₀), which tracks turbidity over time. Measured using a spectrophotometer, OD₆₀₀ provides a rapid assessment of growth rate changes, such as faster or slower doubling times. However, it cannot differentiate between viable and dead cells. This measurement can be taken at predefined incubation times to represent specific growth phases (exponential, stationary, or death) or at regular intervals (minutes or hours) to construct a growth curve, revealing adjustments in lag phase duration or generation time during exponential growth.
To assess viability, colony-forming units per milliliter (CFU/mL) must be determined, quantifying only viable cells capable of forming visible colonies. This requires performing serial dilutions, plating on appropriate agar, and counting colonies. An increase in CFU may indicate enhanced survival or adaptation, while a decrease could suggest environmental stress.
- Changes in phenotype
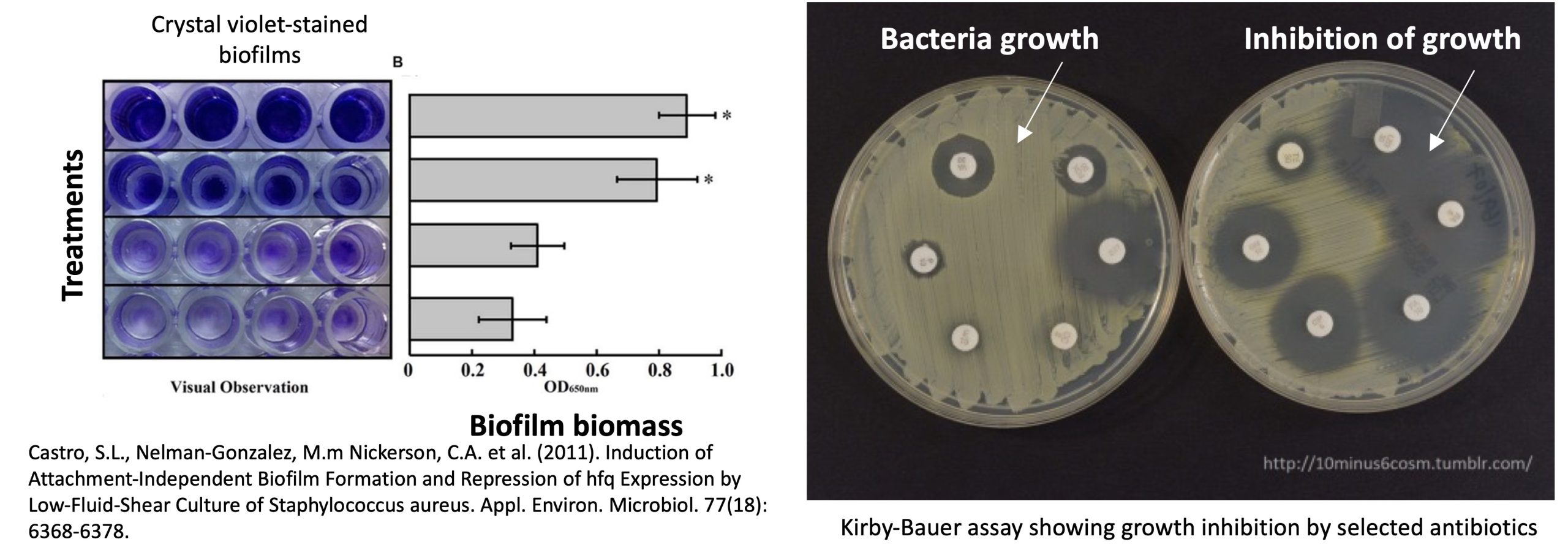
Gravity influences bacterial swimming patterns and flagellar function, hence changes in motility and flagellar structure could be tracked using video microscopy or soft agar motility assays to determine any shift in motility or directional changes due to altered fluid shear forces. Another phenotype aspect commonly altered by microgravity is the formation and structure of biofilms, altered by changes in cell physiology leading to a higher production of extracellular polymeric substances (EPS) and to the alteration of fluid shear. Biofilm biomass can be measured using the crystal violet assay, while structure is generally studied with confocal laser scanning microscopy (CLSM).
- Antibiotic susceptibility & stress responses
The Minimum Inhibitory Concentration (MIC) assay is essential to evaluate how microgravity alters antibiotic resistance and it is generally done using broth dilution or disc diffusion assays with various antibiotics. The expected outcome is a significant change in resistance, potentially due to cell wall modifications or efflux pump activation. Additionally, stress-response gene expression can be analyzed through qPCRor RNA-Seq as bacteria activate stress pathways under microgravity. This experiment requires the extraction of total RNA to quantify the expression of genes related to oxidative stress (sodA, katG), DNA repair (recA, lexA), and antibiotic resistance (efflux pump genes such as acrB, mexAB).
- Transcriptomic and proteomic analyses
Differential gene expression analysis using RNA-Seq helps identify genome-wide metabolic shifts under microgravity by comparing the gene expression profiles between bacteria grown under microgravity and gravity conditions. This approach directly quantifies the number of gene copies coding for proteins related to cell division, metabolism, and virulence, shedding light on microbial adaptation mechanisms. Similarly, studying protein expression and post-translational modifications through mass spectrometry-based proteomics provides further insights into the synthesis of proteins involved in stress-related and biofilm-associated processes, highlighting potential survival strategies in altered gravitational environments.
Want to learn more about microgravity simulation for bacteria and cell cultures? Here are some suggested readings:
Hicks J, Topolski C, Chavez AA, Castillo HA. 2023. Use of a microgravity analog to explore the effects of simulated microgravity on the development of Escherichia coli K12 biofilms. J Microbiol Biol Educ.24:e00062-23. https://doi.org/10.1128/jmbe.00062-23
Nickerson CA, Ott CM, Wilson JW, Ramamurthy R, Pierson DL. Microbial responses to microgravity and other low-shear environments. Microbiol Mol Biol Rev. 2004 Jun;68(2):345-61. doi: 10.1128/MMBR.68.2.345-361.2004. PMID: 15187188; PMCID: PMC419922.
Green MJ, Aylott JW, Williams P, Ghaemmaghami AM, Williams PM. Immunity in Space: Prokaryote Adaptations and Immune Response in Microgravity. Life. 2021; 11(2):112. https://doi.org/10.3390/life11020112
Low Earth Orbit (LEO) refers to the region of space between approximately 160 km (99 mi) and 2,000 km (1,242 mi) above Earth's surface. It is the closest orbital range to Earth and is where most satellites, the International Space Station (ISS), and many space missions operate.
A parabolic flight is a type of aeronautical maneuver used to create short periods of microgravity (weightlessness) by flying an aircraft along a parabolic trajectory. This technique is commonly used for astronaut training, scientific research, and testing space technologies in reduced gravity conditions.
A signaling pathway is a series of molecular interactions within a cell that transmits a signal from outside the cell (or within it) to trigger a specific cellular response. These pathways are essential for processes like growth, metabolism, immune responses, and communication between cells.
Shear stress (τ) is the force per unit area parallel to a surface, causing layers of a material (solid, liquid, or gas) to slide against each other. It is a key concept in fluid dynamics, material science, and biomechanics.
Low-shear fluid conditions refer to environments where fluid flows with minimal tangential force, meaning the velocity gradient between adjacent fluid layers is very small. These conditions are commonly found in microgravity, certain biological environments, and specialized industrial processes.
A bioreactor is a controlled system designed to support biological reactions by providing an optimal environment for the growth of microorganisms, animal cells, plant cells, or enzymes. These systems are widely used in biotechnology, pharmaceuticals, food production, and environmental engineering.
Bacterial doubling time, also known as generation time (g), is the time required for a bacterial population to double in number under optimal growth conditions. It is a key parameter in microbiology, influencing bacterial growth curves and infection dynamics.
A Colony-Forming Unit (CFU) is a measurement of viable bacterial or fungal cells in a sample, based on their ability to form distinct colonies on a solid growth medium. Since some bacteria exist in clusters or chains, CFU represents the number of viable cell groups, not individual cells.
Extracellular Polymeric Substances (EPS) are a complex mixture of polysaccharides, proteins, nucleic acids, and lipids secreted by microorganisms (mainly bacteria and fungi) into their external environment. EPS plays a crucial role in biofilm formation, microbial adhesion, and protection.
Quantitative PCR (qPCR), also known as real-time PCR, is a molecular biology technique used to amplify and quantify DNA or RNA in real-time. Unlike conventional PCR, which only amplifies DNA, qPCR provides quantitative data by measuring fluorescence emitted during amplification.
RNA sequencing (RNA-Seq) is a high-throughput sequencing technique used to analyze the entire transcriptome (all RNA molecules) of a biological sample. It provides insights into gene expression, alternative splicing, transcript isoforms, and non-coding RNA.
